We are a Miami research center leader
Site Capabilities

We have access to:
- Plethysmograph
- Bronchoscopy Center
- Endoscopy Center
- High Resolution CT Scan (64 slice)
- MRI
- PET Scan
- DEXA Scan
- Fluoroscope
- Echocardiography
- Holter Monitoring
- Treadmill Stress Testing
- ABPM
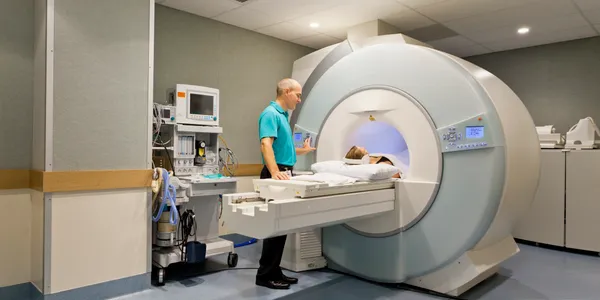
On-Site Equipment:
- ECG Equipment
- Laboratory Processing Equipment, including a Refrigerated Centrifuge and a regular Centrifuge
- Automatic Blood Pressure Cuff
- Weight Scales
- Freezers/Storage
- Investigational Product Double Locked Storage
- Temperature Controlled -20* Freezer
- Temperature Controlled Refrigerator
- Digital Temperature Hourly recording device for all equipment
- Digital Temperature Hourly recording device for Ambient
- Off-Site Record Archives
- Wireless Internet
- Fax
- Scanner
- Color Printer
- Designated Monitoring Rooms

Clinical research is research conducted with human subjects, or material of human origin, in which the researcher directly interacts with human subjects. Clinical research helps doctors and researchers to find new and better ways to understand, detect, control, and treat illness. A clinical research study is a way to find answers to difficult scientific or health questions. For example, the study might explore the best ways to treat people with colon cancer. By studying cancer cells from patients, researchers may be able to determine the specific genetic mutations (changes in gene sequence) that caused the normal, healthy cells to become cancerous, and may help doctors decide on the best drugs to prescribe or surgeries to perform. Clinical research today may help other doctors in the future screen their healthy patients before they ever develop cancer.
A clinical trial is a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
Clinical research is a vital part of finding new treatments and cures for diseases. Carefully conducted clinical studies are the fastest way to find treatments that are safe and effective. By volunteering for a clinical study, you would be participating in research that may result in a new treatment for a deadly or debilitating disease.
Before you agree to participate in a study, you must be given complete information about the study, known as “informed consent.” Informed consent involves two essential components: a document and a process. The informed consent document gives a summary of the research project (including the study’s purpose, research procedures, potential benefits and risks, etc.) and explains the individual’s rights as a research participant. This document is part of an informed consent process, which consists of conversations between the research team and the participant, and may include other supporting material such as study brochures. The informed consent process provides research participants with ongoing explanations that will help them make informed decisions about whether to begin or continue participating in the research project (See: Informed Consent for Genomics Research). The Food and Drug Administration (FDA) provides details about informed consent with the information page: Informed Consent for Clinical Trials [fda.gov].
Because participating in a clinical study is an important decision, there are many questions that you should consider before agreeing to participate. The Centers for Disease Control and Prevention (CDC) have prepared a list of questions to help you get the information you need to make a decision about participating: Taking Part in Research Studies: What Questions Should You Ask? [cdc.gov].


